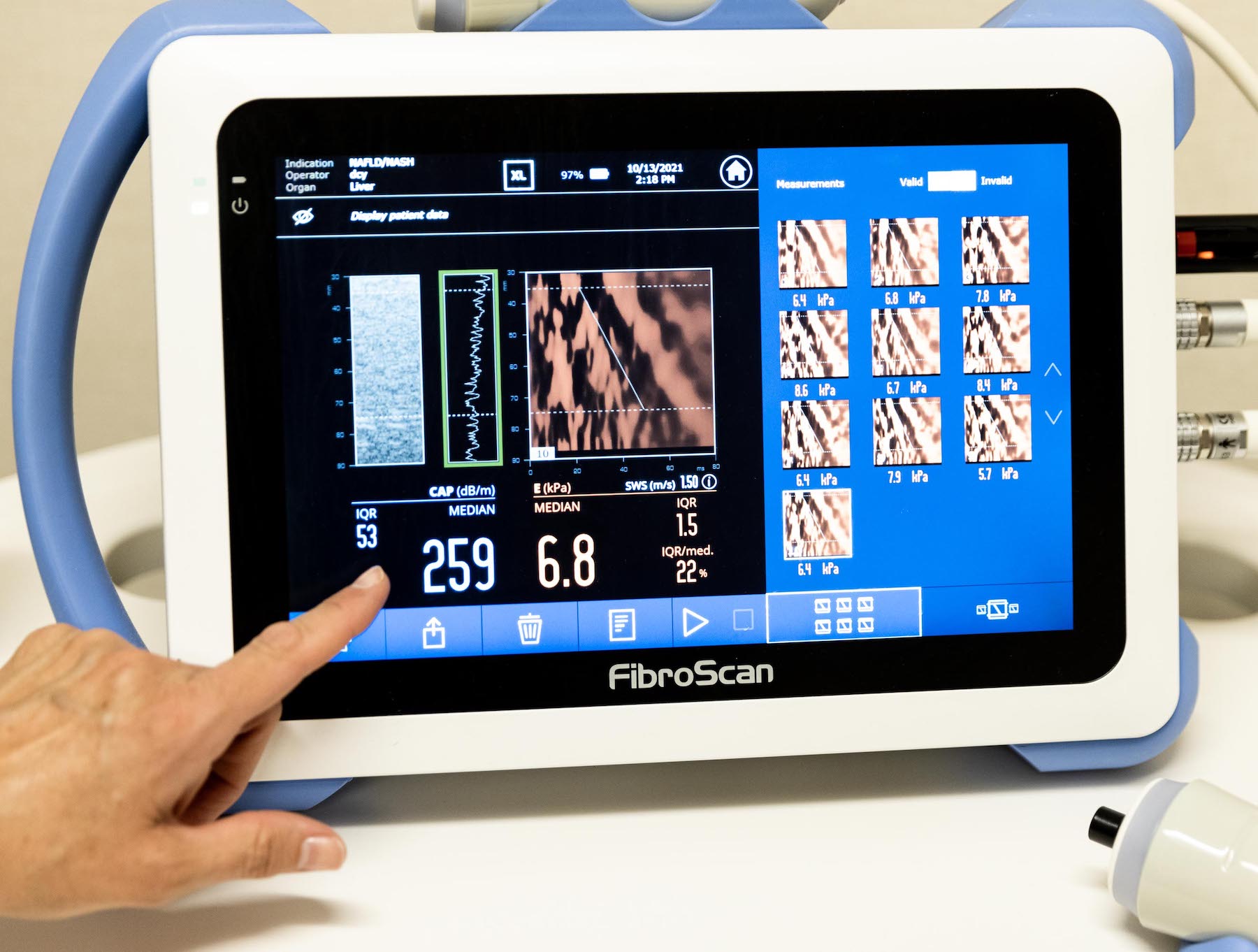
What KCRI is working on
What is a Clinical Trial?
How it Works
Prior to starting a clinical trial, the FDA requires that the medication or device undergo initial testing in animals to test safety and efficacy before moving to human testing. Once human testing has begun, there are 4 stages, or phases, of treatment. Phase I trials are dose finding trials that are conducted in smaller populations. Phase II trials focus on patient safety and work to identify potential side effects with the treatments. Phase III trials are large trials conducted on a few thousand patients. The main focus of these trials is to gather more information on the effectiveness of the drug and monitor additional safety components prior to its presentation to the FDA for approval. Phase IV, the final stage, looks at a more diverse population after the FDA approves the medication.
Clinical trials are conducted according to a plan called a protocol. The protocol provides continuity for the trial and outlines how it will be conducted. It lets the study teams know what tests and procedures to conduct at each visit and provides them with information on how to identify potential side effects. The protocol also guides the study team on how to collect the data needed to evaluate the therapy. The protocol is written using guidelines set by the FDA and Institutional Review Board (IRB) to keep participants safe.
While each trial is different in how they are conducted, the processes still have similarities.
- Informed consent
- Screening process (typically 2-12 weeks)
- Study procedures (i.e. imaging, labs, biopsy)
- Start of therapy (randomization)
- Treatment period (varies depending on trial phase)
- End of treatment
- Follow-up
Informed Consent
Volunteers who participate in a trial must agree to the terms outlined in the study protocol. They do this by reading and signing a document called an informed consent. The informed consent outlines what it means to participate and the process you are agreeing to when you join a study. Participants can withdraw consent from a trial at anytime should they no longer wish to participate.
Kansas City Research Institute
Benefits
Why are clinical trials done?
- Advance medical knowledge
- Promote new and promising treatments
- Facilitate access to otherwise unobtainable medications
- Yield data that empowers both medical professionals and patients to make informed healthcare decisions
There may be many reasons to conduct a clinical trial, including to determine whether a new drug is safe and effective.
Additionally, trials study alternative ways to use and improve existing treatments. They also help develop safe methods for treatment administration for populations in which the treatment was not previously tested.